
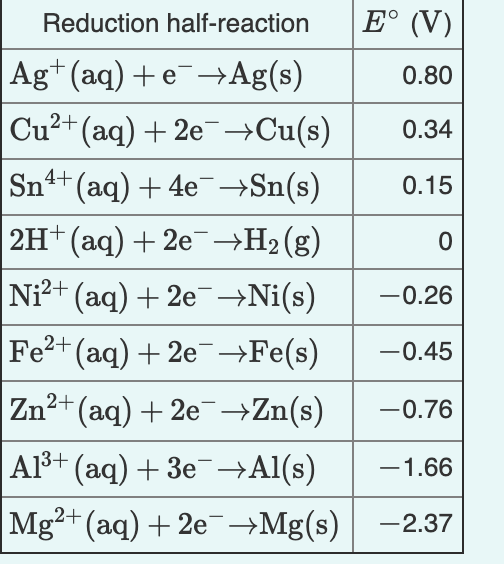
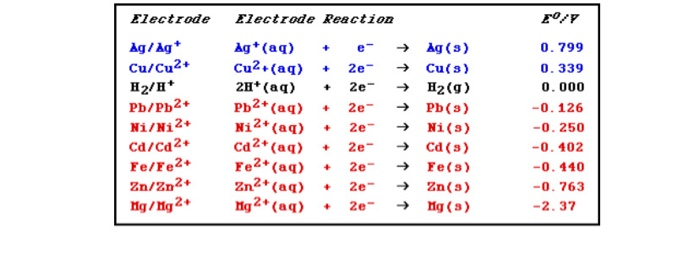
Given that,' ENi^2 + / Ni^∘ = - 0.25 V, ECu^2 + / Cu^∘ = + 0.34 V EAg^2 + / Ag^∘ = + 0.80 V, EZn^2 + / Zn^∘ = - 0.76 V Which of the following redox processes will not take place in specified direction?

Impacts of Dissolved Ni2+ on the Solid Electrolyte Interphase on a Graphite Anode - Xu - 2022 - Angewandte Chemie International Edition - Wiley Online Library

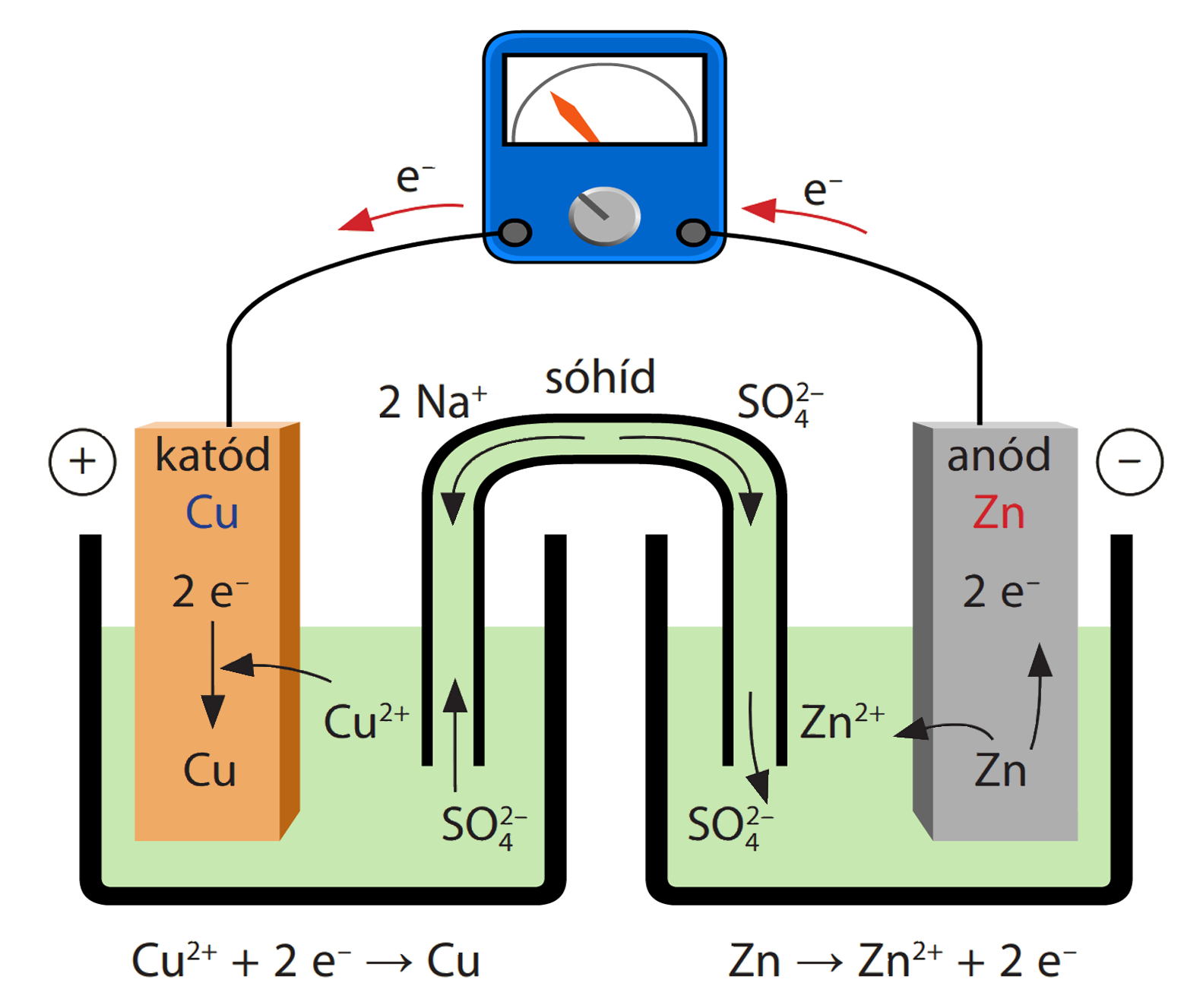



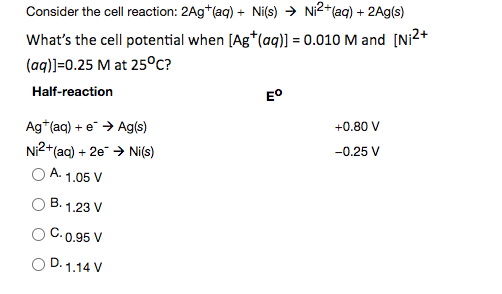

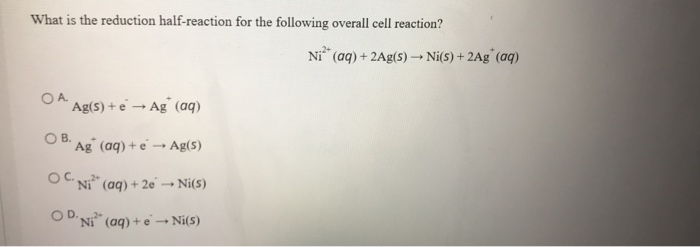

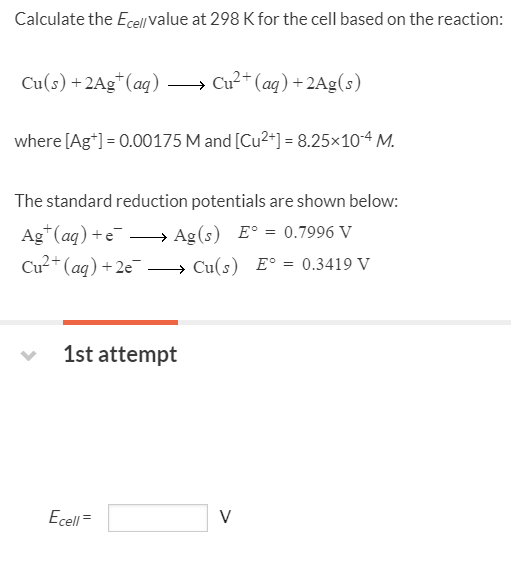
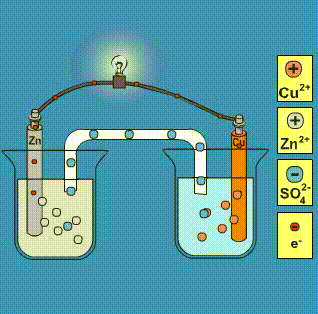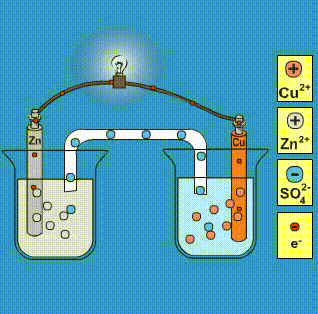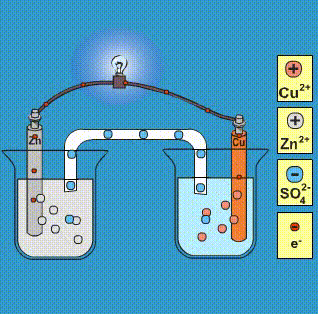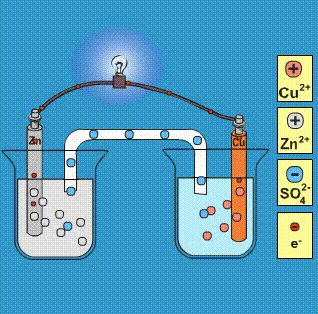
-ion.jpg?ezimgfmt=rs:363x187/rscb1/ngcb1/notWebP)
