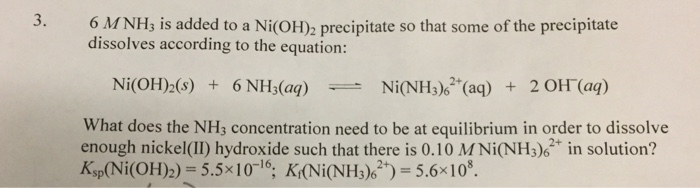Nickel-based alloy corrosion in CANDU steam generators: E-pH diagrams of the Ni-NH3-H2O and Ni-CH3COO -H2O ternary systems

Nickel transition metal Chemistry nickel(II) Ni2+ complex ions ligand substitution redox chemical reactions principal oxidation states +2 +3 GCE AS A2 IB A level inorganic chemistry revision notes

Amongst the following, the total number of compounds soluble in concentrated NH3 solution is: Ag2CrO4,Cu(OH)2, PbSO3, Al(OH)3, Ni(OH)2, Zn3(PO4)2,BaSO4

Electrocatalytic ammonia oxidation over a nickel foam electrode: Role of Ni( OH)2(s)-NiOOH(s) nanocatalysts - ScienceDirect

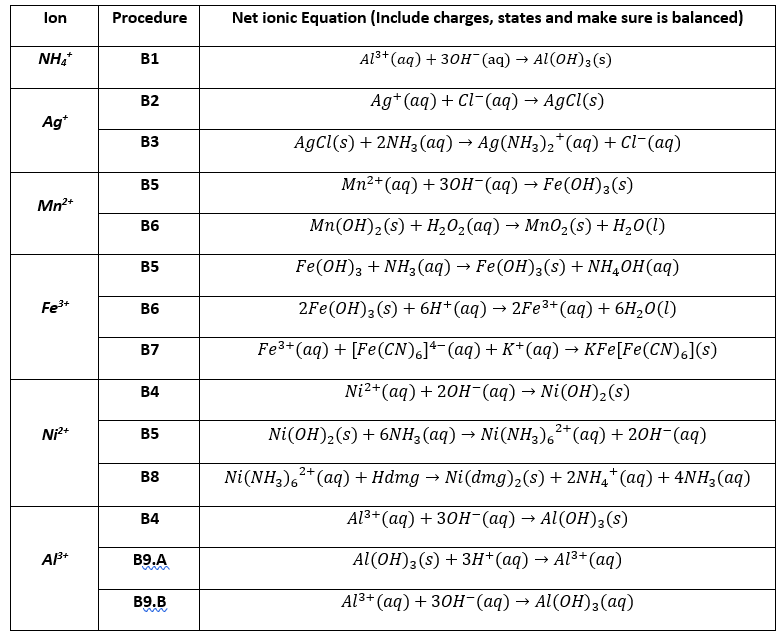
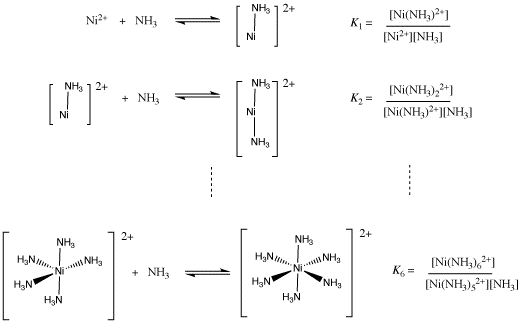
SOLVED: Questions Write net ionic equations for the reaction of nickel(ll) chloride with 6M NaOH t0 give Ni(OH)z: the reaction of iron(III) nitrate with 6M NaOH to give iron(IlI) hydroxide The reaction

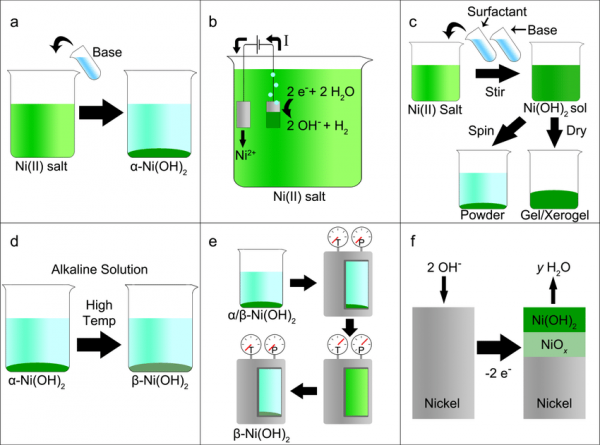
Triple Functions of Ni(OH)2 on the Surface of WN Nanowires Remarkably Promoting Electrocatalytic Activity in Full Water Splitting | ACS Catalysis

SEM images of various electrocatalysts synthesized in water and ammonia... | Download Scientific Diagram

Nickel transition metal Chemistry nickel(II) Ni2+ complex ions ligand substitution redox chemical reactions principal oxidation states +2 +3 GCE AS A2 IB A level inorganic chemistry revision notes

Electrocatalytic ammonia oxidation over a nickel foam electrode: Role of Ni( OH)2(s)-NiOOH(s) nanocatalysts - ScienceDirect

Inorganics | Free Full-Text | Ni(NH3)2(NO3)2—A 3-D Network through Bridging Nitrate Units Isolated from the Thermal Decomposition of Nickel Hexammine Dinitrate

Porous Fe-Doped β-Ni(OH)2 Nanopyramid Array Electrodes for Water Splitting | ACS Applied Materials & Interfaces

E735: Complex Ions and Precipitates – Nickel(II) compounds | Lecture Demonstration Manual General Chemistry | University of Colorado Boulder